COVID-19 pandemic has spread through the globe and the death count goes higher day by day. Expertise in the areas of Medical, Science, Biotechnology has been developing remedies for survival, and as a result, the Covid-19 Vaccines, a liquid that many hope could help to end the pandemic came into life. While the circumstances were like this, ModernaTX Inc., a 10-year-old biotech company, is racing forward with a vaccine of its own by using a once dismissed idea; the mRNA technology.
Basic information on Moderna
The Moderna COVID‑19 vaccine, code-named mRNA-1273 is a COVID-19 vaccine developed by ModernaTX Inc., the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). It is authorized for use for people 18 years and older in most jurisdictions to provide protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus.
The vaccine is designed to be administered as two 0.5 mL doses to the muscle of the upper arm at an interval of at least 28 days apart and some immunocompromised people should get 3 shots.
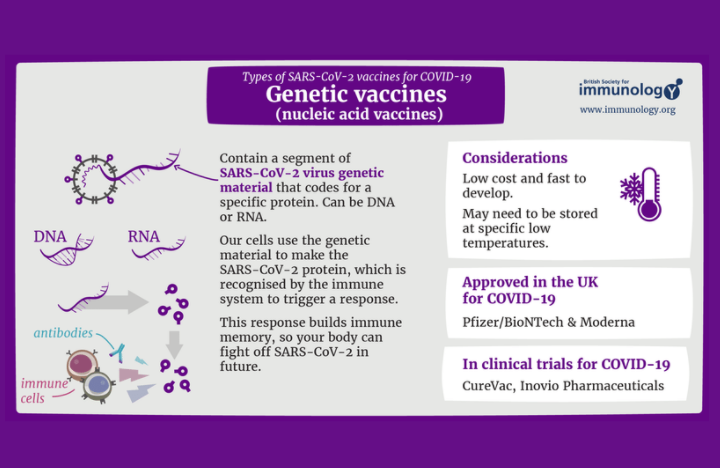
Who should be vaccinated?
As with all COVID-19 vaccines, health workers at high risk of exposure and older people are prioritized for vaccination. Furthermore, WHO recommends the use of Moderna in pregnant women when the benefits of vaccination to the pregnant woman outweigh the potential risks. The FDA approved a booster dose (the third dose) of the Moderna vaccine for certain immunocompromised individuals, including solid organ transplant recipients and those with conditions that give them an equally reduced ability to fight infections and other diseases.

However, individuals with a history of severe allergic reactions to any component of the vaccine should not take this or any other mRNA vaccine. The vaccine should not be administered to persons younger than 18 years of age as results of further studies are pending.
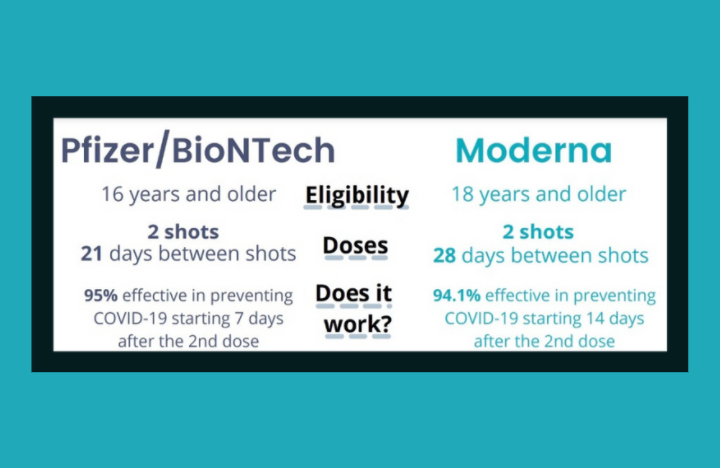
Is it safe?
Is it actually safe? This is the main concern of all COVID-19 vaccines as the vaccines were developed within a short period of time with fewer clinical trials compared to other vaccines. Also, the lack of time has made it impossible to identify the side effects. Especially the long-term ones. However, on 30 April, WHO listed the Moderna vaccine for emergency use. The EMA has thoroughly assessed the data on the quality, safety, and efficacy of the Moderna COVID-19 vaccine and authorized its use across the European Union. Moderna’s vaccine was authorized for emergency use in the U.S. in December 2020, about a week after the Pfizer vaccine.
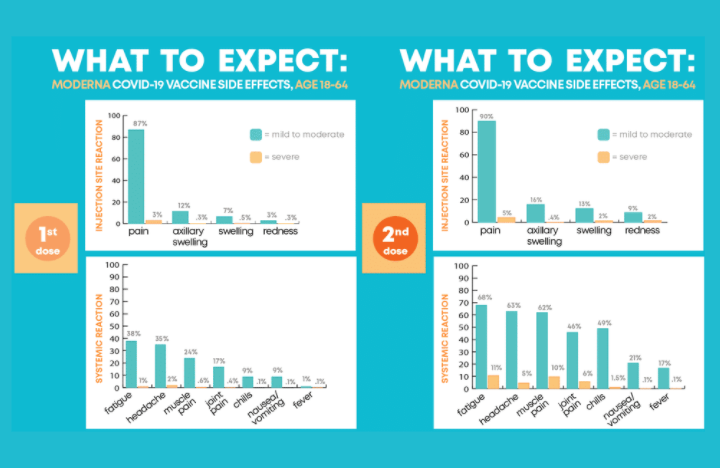

Efficacy
As per the WHO it has an efficacy of approximately 94.1% in protecting against COVID-19 which can be considered a very high efficacy level. Based on the evidence so far, Moderna states the new variants of SARS-CoV-2, including the Alpha variant (B.1.1.7) and the Beta variant (501Y.V2 or B.1.351), do not alter the effectiveness of their mRNA vaccine. They also recommend the vaccine against the Delta variant (B.1.617.2) although it did show to be about two times weaker against Delta than against the original virus.
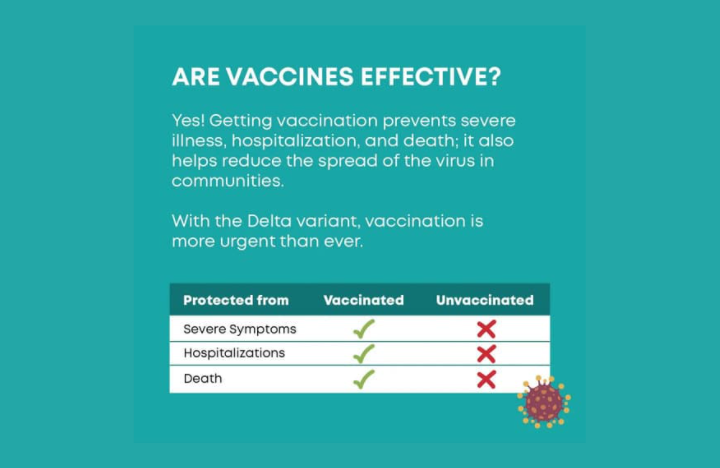
The emergence of new variants is most likely to happen with the mutation of the virus and only the clinical studies can tell how the effectiveness of the Moderna vaccine will be. But it is our responsibility as sensible citizens to get vaccinated or if by any chance you choose not to get vaccinated, given the circumstances, you need to act wisely to not spread the disease.
Written by Rtr. Nethmi Ranasinghe

Very informative!